orange book pharmacy definition
_ GITAM Institute of Pharmacy. An introduction a how to use section the drug product lists appendices and a patent and exclusivity information addendum.

Introduction To Immunology Stomp On Step1 Immunology Pharmacy Books Immunity
The Orange Book contains therapeutic equivalence evaluations for approved multisource prescription drug products usually referred to as generics.
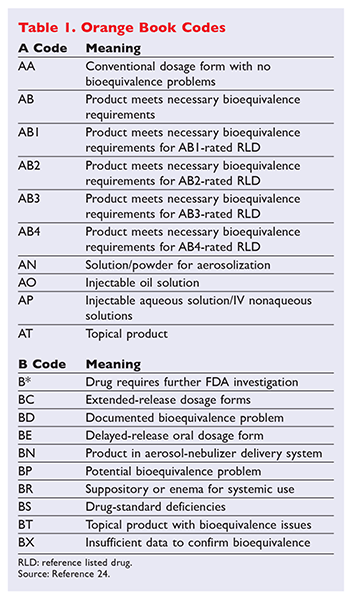
. THE ORANGE BOOK thLecture Notes_Dr. The Orange Book is an important publication published by the FDA that serves as the gold standard reference for generic drug substitution. Page 1 Definition It is the publication of Approved Drug.
Objectives What is a Generic Drug. 1 approved prescription drug products with therapeutic equivalence evaluations. R i s k r e.
A The maximum reasonable fee for pharmaceuticals and pharmacy services rendered after January 1 2004 is 100 of the reimbursement prescribed in the relevant Medi-Cal payment system including the Medi-Cal professional fee for dispensing. Orange Book FDA Website Type. Approved Drug Products with Therapeutic Equivalence Evaluations.
Office of Generic Drugs Policy Center for Drug Evaluation Research US. The Orange Book Introduction. The evaluations have been prepared as a resource for state health agencies physicians pharmacists and the public to promote public education in the area of drug product selection as well as to.
The Orange Book Risk Management Principles. Use of unapproved drugs require complete CMC information depending on the nature of the study. Then use the Ingredient Search for.
2 approved over-the-counter OTC drug products for those drugs that. School of Pharmacy 2010 Becker Drive Lawrence KS 66047-1620 Bus Route. Enforce State Pharmacy Boards Oversight of Patient Counseling Laws.
Get emails about this page. Not much more than 30 pages in length this voluntary guide was an aid to manufacturers to understand the needs of the regulatory authoritys requirements for the manufacture of. CDR Kendra Stewart RPh PharmD.
Trusted for more than 120 years RED. Our online pharmacy sells quality products in the USA Canada and around the world. The Orange Book is a compendium of significant unimplemented nonmonetary recommendations for improving departmental operations.
The orange book is a list of generic drugs approved by FDA. 1 Generic substitution laws are state specific and many require use of the Orange Book. The Orange Book is an important publication published by the FDA that serves as the gold standard reference for generic drug substitution.
The orange book consist of five main sections. The 2022 edition of Rules and Guidance for Pharmaceutical Manufacturers and Distributors the Orange Guide is now available through. The Orange Book has long been a reliable resource for information about FDA-approved drugs.
G o v e r n a n c e and L e a d e r s I n te g ra o n h i p C o l a b or ti o n Information Insight Insight Information Communication. The Orange book has been revised. Before understanding different drug ratings it is necessary.
This determines the ingredient s. 65 Strengthen FDA. FDA orange book The official name of FDAs orange book is Approved Drug Products with Therapeutic Equivalence Evaluations.
Basics in drug approval process with reference to the Orange Book Presented by. First if you have the trade name search the Electronic Orange Books Rx or OTC section using the Proprietary Name search. The full publication title is Approved Drug Products with Therapeutic Equivalence Evaluations but it is commonly known as the Orange Book.
Food and Drug Administration. The Office of Inspector General. The electronic availability of the Orange Book brings this valuable tool to the web for healthcare.
Multicultural Pharmacy Scholars Program. Facebook instagram twitter youtube linkedin. Rucha Pathak Roll No.
At the pharmacy generic substitution is the process by. The full publication title is Approved Drug Products with Therapeutic Equivalence Evaluations but it is commonly known as the Orange Book. First published in 1971 the original Orange Guide contained British Good Manufacturing Practice and was entitled Guide to Good Pharmaceutical Manufacturing Practice.
Updated with Orange Book. The Orange Book is composed of four parts. Rules and Guidance for Pharmaceutical Manufacturers and Distributors commonly known as the Orange Guide brings together all the main European and UK directives regulations and legislation relating to the manufacture and distribution of medicines.
FDAs Approved Drug Products with Therapeutic Equivalence Evaluations Orange Book identifies drug products approved on the basis of safety and effectiveness. Orange Book FDA Link. Sumanta Mondal_MPharm 1 Sem.
Sciences College of Pharmacy 10920 S Riverfront Parkway Utah 84095 South Jordan USA Tel 801-878-1078 Email vkalerosemanedu. Formally known as Approved Drug Products with Therapeutic Equivalence Evaluations the orange book lists drugs which are not only safe but also effective for human use. FDA has draft guidance explaining that certain currently marketed drug ingredients were marketed before current FDA legislation.
Sponsors using these products should consult FDA about the need for an IND. The FDAs Orange Book identifies approved drug products.

The Introduction Of An Orange Book
Appendix A Medical Terminology And Abbreviations In Manual For Pharmacy Technicians
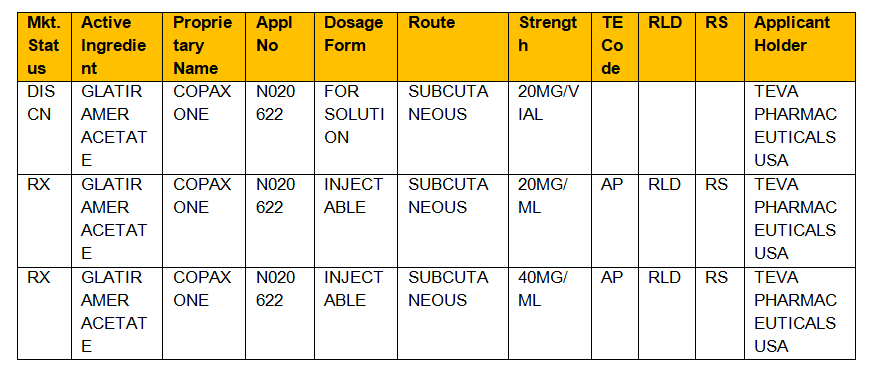
Orange Book And Its Applications Legal Advantage

The Introduction Of An Orange Book

Pharmaceutical Marketing Strategies

The Introduction Of An Orange Book

Investigational New Drug Orange Book Understanding On 505 B 2 A

Insights Into Effective Generic Substitution

Therapeutic Equivalence Codes Effects Substitution Video Lesson Transcript Study Com
Regulatory 101 Drug Name Modifiers Definition Categories Generics And Capa Ivt

The Introduction Of An Orange Book

The Introduction Of An Orange Book
/doctor-826e0c116cd549d98e327f1184c622d9.jpg)